Abstract
Introduction
Hematological cancer patients are often vitamin C deficient, and vitamin C is essential for the TET induced conversion of 5-methylcytosine (5mC) to 5-hydroxymethylsytosine (5hmC); the first step in active DNA demethylation. In tissue culture, we have shown that restoration of vitamin C to normal concentrations can potentiate the effect of DNA methyltransferase inhibitors (DNMTis) by activation of DNA demethylation and induction of genes in the viral defense pathway, so called 'viral mimicry' (Liu et al. PNAS 2016). Here, we investigated whether oral vitamin C supplementation can correct vitamin C deficiency, enhance the efficacy of active DNA demethylation and induce upregulation of genes in the viral defense pathway in patients with myeloid cancers treated with the DNMTi 5-azacytidine.
Study Design and Methods
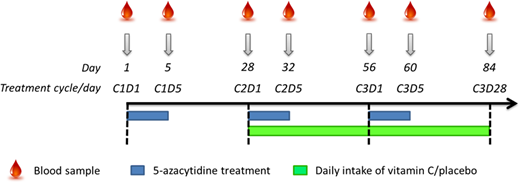
A randomized, placebo-controlled clinical trial of myelodysplastic syndrome (MDS; n=9), chronic myelomonocytic leukemia (CMML; n=4) and acute myeloid leukemia (AML; n=7) patients was performed during 3 cycles/12 weeks of DNMTi treatment (5-azacytidine, 100mg/m2 day 1 to 5 in a 4-week cycle) supplemented by oral dose of 500mg vitamin C (n=10) or placebo (n=10) daily during the last 8 weeks (Figure 1). Blood samples were drawn on day 1 and 5 of each treatment cycle before 5-azacytidine was administered and on day 28 of the third treatment cycle. Total plasma vitamin C was measured by HPLC in samples that had been acidified by 10% meta-phosphoric acid immediately after blood drawn. Mutational status of the 20 most commonly mutated genes in MDS were conducted by targeted next-generation sequencing. Global levels of 5mC and 5hmC were measured with LC-MS/MS in DNA extracted from MACS-sorted malignant cells and quoted relative to total levels of deoxyguanine. Total RNA-seq was performed on 20 RNA samples from 6 patients; cDNA libraries were prepared using KAPA RNA HyperPrep Kits and sequencing on a NextSeq 500 instrument (Illumina).
Results
Fourteen patients were deficient in plasma vitamin C (<23 µM) and 4 of the remaining 6 patients took vitamin supplement at inclusion. Global DNA methylation was significantly higher in patients with severe vitamin C deficiency (<11.4 µM; P=0.004) and borderline significantly higher in DNMTi naïve (n=11) compared to non-naïve patients (P=0.095). At baseline, global 5hmC/5mC levels were lower in the 7 patients with TET2 mutations (n=7; P= 0.013). Oral supplementation restored plasma vitamin C levels to the normal range in all patients in the vitamin C arm (P<0.0005). We show for the first time that active DNA demethylation, estimated as the change in global 5hmC/5mC levels, was significantly increased in patients receiving vitamin C compared to placebo (P=0.041). Preliminary RNA sequencing data show increased upregulation of genes involved in the viral defense pathway, including IRF7 and IFIT1, in vitamin C supplemented patients that were DNMTi naïve at study inclusion.
Conclusions
The increase in active DNA demethylation, indicated by the elevation of 5hmC/5mC levels in myeloid cells from vitamin C supplemented patients compared to placebos, plus the increased expression of viral defense genes in vitamin C treated DNMTi naïve patients, suggest that the efficacy of 5-azacytidine might be increased by oral supplementation of vitamin C. We suggest that normalization of plasma vitamin C by oral supplementation may enhance the biological effects of DNMTis in patients and prompts the investigation of the clinical relevance of vitamin C supplementation to DNMTis in a large randomized placebo controlled trial.
Figure 1. Study design. Days 1, 5, and 28: before vitamin C/placebo exposure. Day 32: after short-term vitamin C/placebo exposure. Days 56, 60, and 84: after longer-term vitamin C/placebo exposure. C = cycle, D = day in cycle.
Jones:ZYMO Corporation: Consultancy. Grønbæk:Celgene: Membership on an entity's Board of Directors or advisory committees; Otsuka Pharma: Membership on an entity's Board of Directors or advisory committees; Janssen Pharma: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal